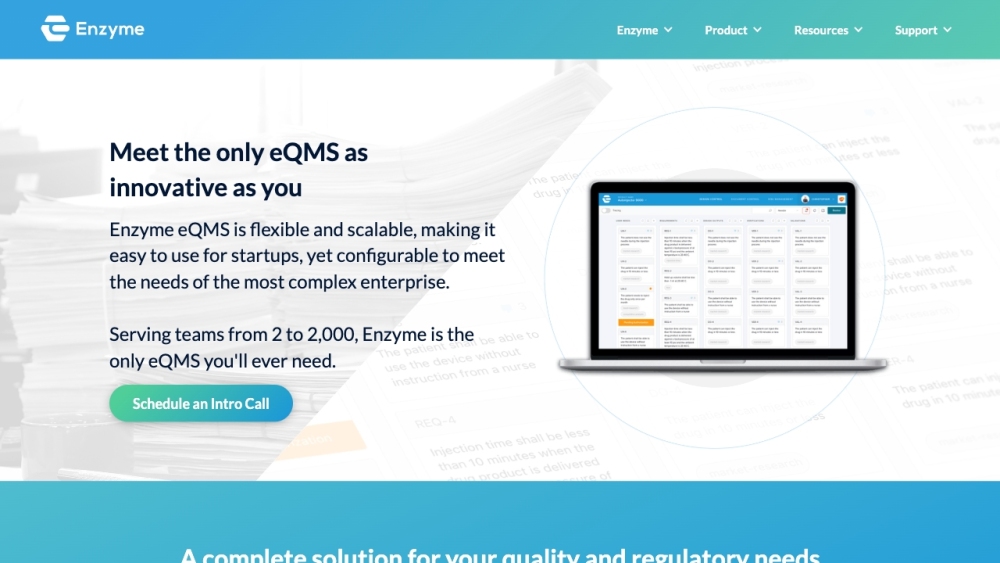
Enzyme
Enzyme software assists medical organizations in maintaining quality and adhering to industry regulations.
Alternative Tools
scrol.ai
Utilize scrol.ai to engage in conversations, search for information, and produce data rapidly using Artificial Intelligence technology.
Text&Writing
Marketing
FlowGPT
FlowGPT offers a collection of varied prompts for ChatGPT, designed to improve conversation.
Other
MightyGPT
MightyGPT utilizes GPT-3 technology and chatGPT to engage in conversations on messaging platforms, providing assistance to users.
Chatbot